Research
Oligodendrocytes are glial cells of the central nervous system (CNS) that interact with axons and form myelin membranes to enable saltatory conduction. Oligodendrocyte abnormality or loss from injury can negatively impact axonal integrity and CNS function, leading to axonal dystrophy and neurodegeneration such as that observed in multiple sclerosis (MS), spinal cord and traumatic brain injuries. The questions that we seek to understand are:
To answer these questions, we are using primary oligodendrocyte and neuron cultures, transgenic mice, and models of experimental CNS demyelination, combined with molecular biology and imaging tools.
- How do oligodendrocytes and myelin regenerate after injury?
- How do oligodendrocytes influence neuronal integrity and survival?
To answer these questions, we are using primary oligodendrocyte and neuron cultures, transgenic mice, and models of experimental CNS demyelination, combined with molecular biology and imaging tools.
Myelin Regeneration
Myelin regenerates spontaneously after demyelination. This is a process called "remyelination". For remyelination to be successful, cellular debris from tissue injury must be removed, and new oligodendrocytes must be available to replace myelin. These events require a sequence of well-controlled signals that regulate inflammation and endogenous neural stem/precursor cell mobilization and differentiation to restore form and function to the CNS.
|
|
The major players of remyelination are (1) oligodendrocyte precursor cells (OPCs), which are widely distributed neural progenitors in the adult CNS that are able to differentiate into oligodendrocytes, (2) microglia, which are immune-surveillance cells in the adult CNS that are needed for debris clearance and regulation of inflammation, (3) axons, which provide the signal(s) and membrane surface for oligodendrocytes to interact with and synthesize myelin, and (4) astrocytes, which form a structural barrier (scar) around the lesion and influence inflammation.
After CNS demyelination, remyelination is achieved by the coordinated expression of regenerative signals at the lesion that promotes inflammation and OPC proliferation and migration, followed by signals that decrease inflammation and induce oligodendrocyte differentiation and myelin synthesis. We have identified many of these signals from a microarray analysis of CNS remyelination (Huang et al., 2011), and are currently investigating their roles in remyelination. |
Oligodendrocyte-Axon Interaction
Oligodendrocytes interact with axons in at least two ways. They enwrap axons and form myelin membranes that are necessary for saltatory conduction. They also provide neurotrophic and metabolic support to axons, which are necessary to maintain axonal integrity and promote neuroprotection. For reasons that are poorly understood, chronic oligodendrocyte/myelin loss results in widespread axonal abnormality and degeneration. We are currently investigating the mechanisms that regulate oligodendrocyte interaction with axons, and how oligodendrocytes exert a protective influence on them.
Relevance to Multiple Sclerosis (MS)
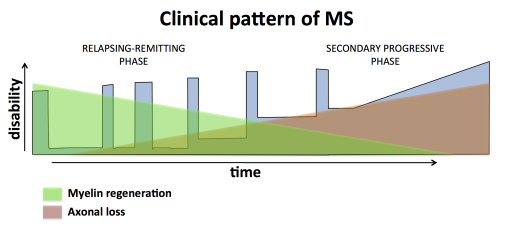
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system (CNS) in which myelin membranes are destroyed through persistent inflammation. Current treatment strategies mainly aim to suppress inflammation, which have been effective in the early stage of the disease (i.e. relapsing remitting phase), but these treatments have not been successful in the later stage of the disease (i.e. secondary progressive phase) where neurodegeneration is a prominent feature. In progressive MS, chronic neurodegeneration correlates with remyelination failure. Since oligodendrocytes and myelin are needed to maintain axonal integrity, failed remyelination is likely a major contributor to the irreversible clinical decline that occurs at the later stage of the disease. Therefore identifying therapeutic strategies to repair demyelinated axons by promoting remyelination and/or neuroprotection would be expected to limit the accumulation of clinical disability and bring disease progression to a halt (Melchor et al., 2019; Huang and Franklin, 2012).
